Disposable syringe pre-fi lled with 4% Tribasic Sodium Citrate. Sterilised in a moist heat autoclave
D.B.M. C-LOCK 4% is a Class IIb CE Medical Device, intended for the safe management of vascular access. Its function is to keep the central venous catheters (CVC) patency in the interdialytic interval, also performing an anticoagulant function in the catheter lumen.
The packaging of the product allows for good conservation and easy storage at temperatures between +5 ° C and +30 ° C.
Validity: 3 years in unopened package.
Code
PCE13KA2
Solution Vol.
2x2,5 mL
Syringe
2x3 mL LL
Quanty/Box
120 pouches/box
240 syringes/box
* The product is also available in other formats.
For more information, contact info.dbm@numantec.eu
Even for sterile fields!
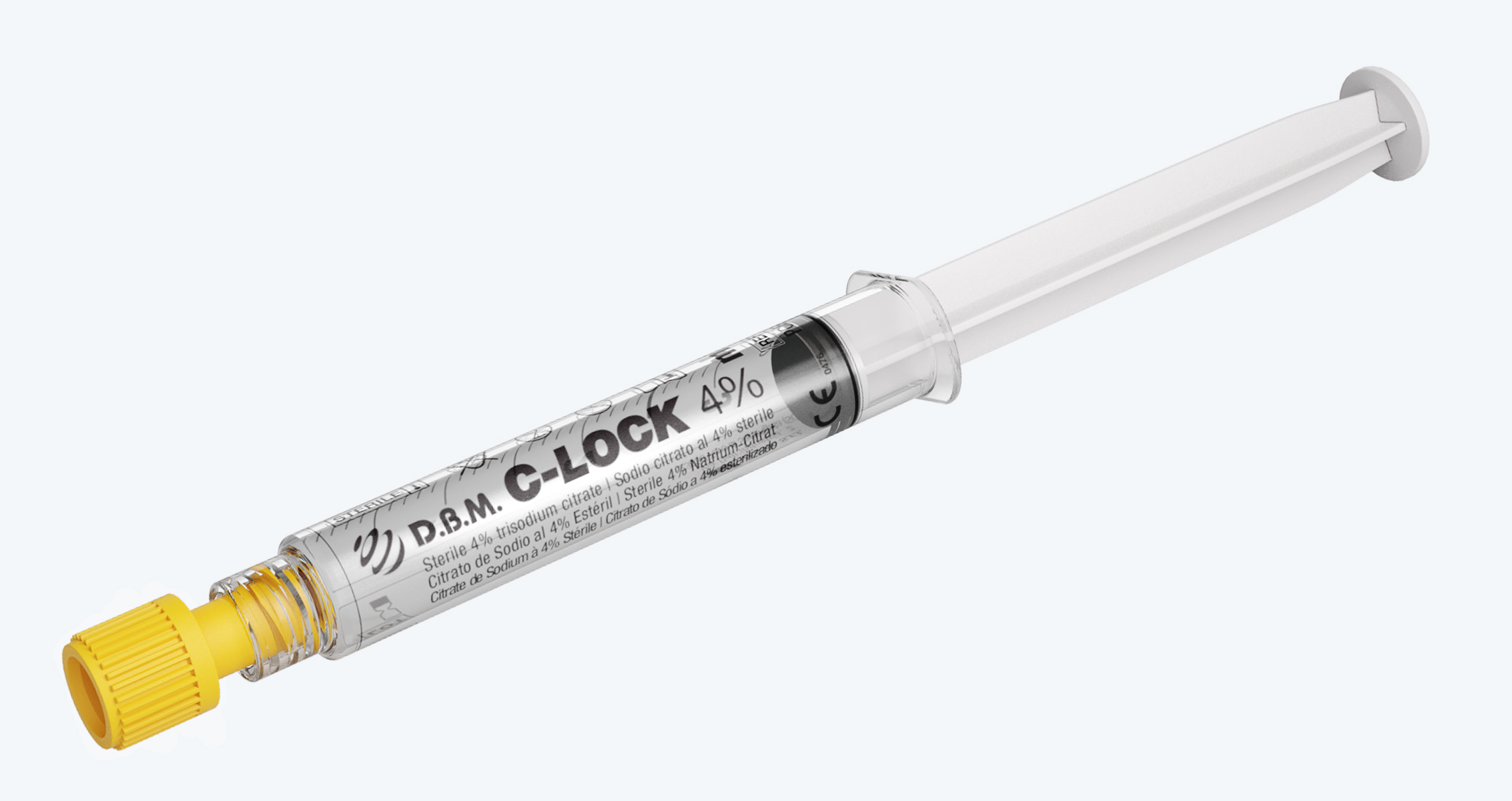
Luer Lock cone
that allows easy
connection with complementary
fittings, thus ensuring
a perfect seal
High transparency
polypropilene syringe
for an excellent view
of the contents
Easy identification
of the product through
the data shown
on the syringe
Latex free rubber
If you are a health professional and need support or would like further information, please request the data sheet
Sterilised in a moist heat autoclave – Latex Free – Single use
Headquarters and Plant DBM1
Via dell’Industria, 2
Warehouse and Plant DBM2
Via dell’Industria, 8
23030 Tovo di Sant’Agata (SO)
Tutti i diritti riservati – D.B.M. S.r.l. a socio unico – Privacy Policy – Cookie Policy – Disclaimer
Design by Minuart
P. IVA / C.f. e iscrizione R.I. di Sondrio 00906360144 – R.E.A. SO-68339 – Capitale Sociale € 21.000,00 I. V. – Società soggetta ad attività di direzione e coordinamento di Delta Med S.p.A
The pages of this website are intended for health professionals only.
The information they contain is to be understood as intended for these users only. Therefore, to visit these pages, I hereby declare under my own responsibility that I belong to the aforementioned professional category.
With reference to the supplement to the circular of 23/03/2006, issued on 20/04/2010 by the Italian Ministry of Health, concerning the advertising of medical devices, only doctors, pharmacists and other health professionals may consult the contents of this website.
1. Information.
This website contains information intended solely for medical doctors and health professionals. D.B.M. Srl shall not be held liable for access by third parties to the information pages for health professionals as specified above. The medical device descriptions presented on these pages are for information purposes only and are not for advertising purposes. The information on this website is of a general nature and is not an alternative to seeking appropriate medical treatment, nor is it intended to advertise any medical procedures. Patients should speak to their doctor to assess treatment options, risks and benefits.
2. Links.
All links on this website are provided for your convenience only. D.B.M. Srl accepts no responsibility for the contents of third-party websites. With regard to the links on its website, D.B.M. Srl expressly excludes any intentions other than that of merely providing information.
3. Intellectual property.
All text, images, drawings and attachments are the exclusive property of D.B.M. Srl. Anyone using them improperly or without authorisation will be prosecuted.