D.B.M. constantly strives to update and improve its product range, and the company’s Research and Development area plays a key role in this.
D.B.M.’s Research and Development department seeks out innovative solutions to create new medical devices or improve existing ones.
This allows it to meet its customers’ requirements with ingenious, cutting-edge solutions.
With a thriving Research and Development area, the company never stops growing. This means that it constantly places new products on the market, while also opening its doors to young new talent.


Producing innovative medical devices that keep pace with advances in healthcare and optimise intervention times calls for continuous investment in cutting-edge technologies.
D.B.M. uses state-of-the-art equipment to improve all stages of product processing, ensuring high quality at all times.
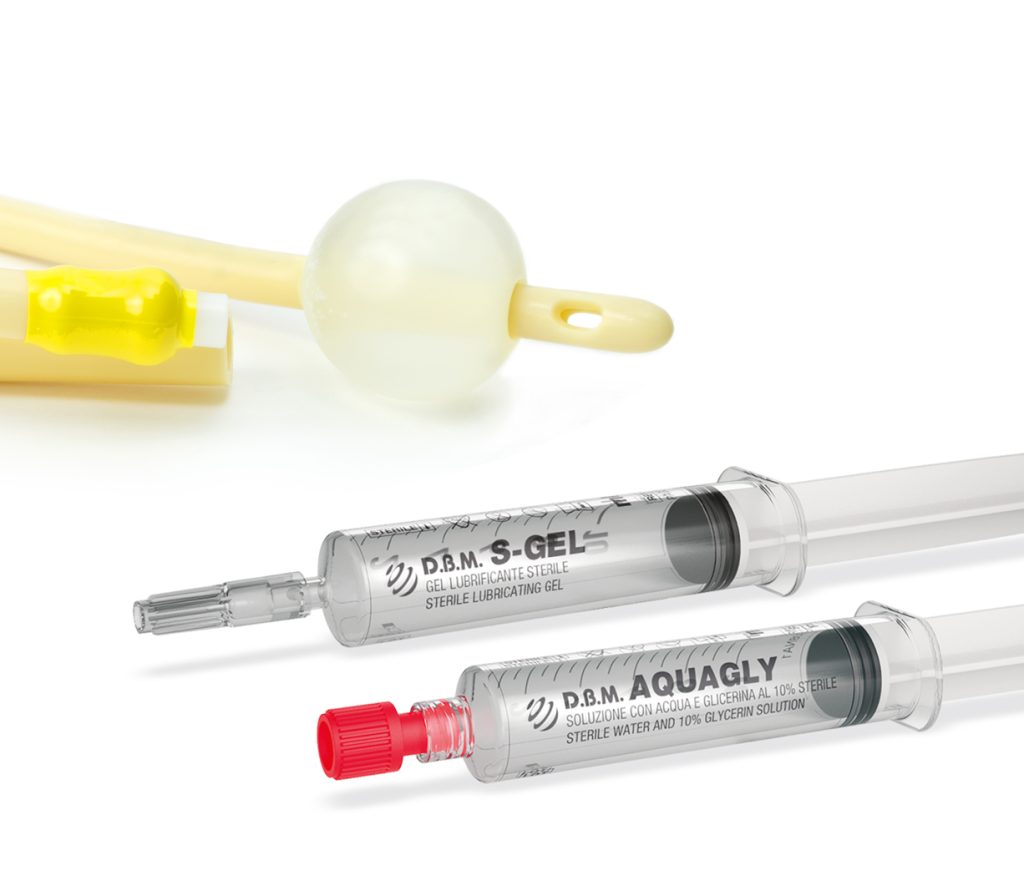
Sterile solutions in syringes for single use are among the most innovative and popular state-of-the-art medical devices.
Their numerous advantages have fuelled a rise in demand from health professionals. However, this growing demand has been met with a shortage in supply.
D.B.M. has successfully bridged this gap by developing its own line of CE-marked pre-filled-syringe medical devices.
Comodità d’uso
Preparation times are significantly reduced.
Traceable solutions
The solution inside the syringe has been checked and analysed.
Improved hygiene
The patient is not exposed to dangerous cross-contamination.
Improved safety
By eliminating glass ampoules and vials, potential injuries to health workers or users are avoided during syringe preparation.
Practical, user-friendly products
Ready-to-use sterile solutions in syringes for single use save time in patient management.

The management team constantly pursues the strategic goal of striving for high quality in the company’s products and the management of the company as a whole.
D.B.M. has a quality management system in place which it updates on a regular basis, along with targeted refresher courses and training. This allows the company to comply thoroughly with applicable international quality standards and regulatory requirements.
It has obtained the UNI CEI EN ISO 13485 Certification: 2016, as a guarantee of quality for its customers.
Headquarters and Plant DBM1
Via dell’Industria, 2
Warehouse and Plant DBM2
Via dell’Industria, 8
23030 Tovo di Sant’Agata (SO)
Tutti i diritti riservati – D.B.M. S.r.l. a socio unico – Privacy Policy – Cookie Policy – Disclaimer
Design by Minuart
P. IVA / C.f. e iscrizione R.I. di Sondrio 00906360144 – R.E.A. SO-68339 – Capitale Sociale € 21.000,00 I. V. – Società soggetta ad attività di direzione e coordinamento di Delta Med S.p.A
The pages of this website are intended for health professionals only.
The information they contain is to be understood as intended for these users only. Therefore, to visit these pages, I hereby declare under my own responsibility that I belong to the aforementioned professional category.
With reference to the supplement to the circular of 23/03/2006, issued on 20/04/2010 by the Italian Ministry of Health, concerning the advertising of medical devices, only doctors, pharmacists and other health professionals may consult the contents of this website.
1. Information.
This website contains information intended solely for medical doctors and health professionals. D.B.M. Srl shall not be held liable for access by third parties to the information pages for health professionals as specified above. The medical device descriptions presented on these pages are for information purposes only and are not for advertising purposes. The information on this website is of a general nature and is not an alternative to seeking appropriate medical treatment, nor is it intended to advertise any medical procedures. Patients should speak to their doctor to assess treatment options, risks and benefits.
2. Links.
All links on this website are provided for your convenience only. D.B.M. Srl accepts no responsibility for the contents of third-party websites. With regard to the links on its website, D.B.M. Srl expressly excludes any intentions other than that of merely providing information.
3. Intellectual property.
All text, images, drawings and attachments are the exclusive property of D.B.M. Srl. Anyone using them improperly or without authorisation will be prosecuted.